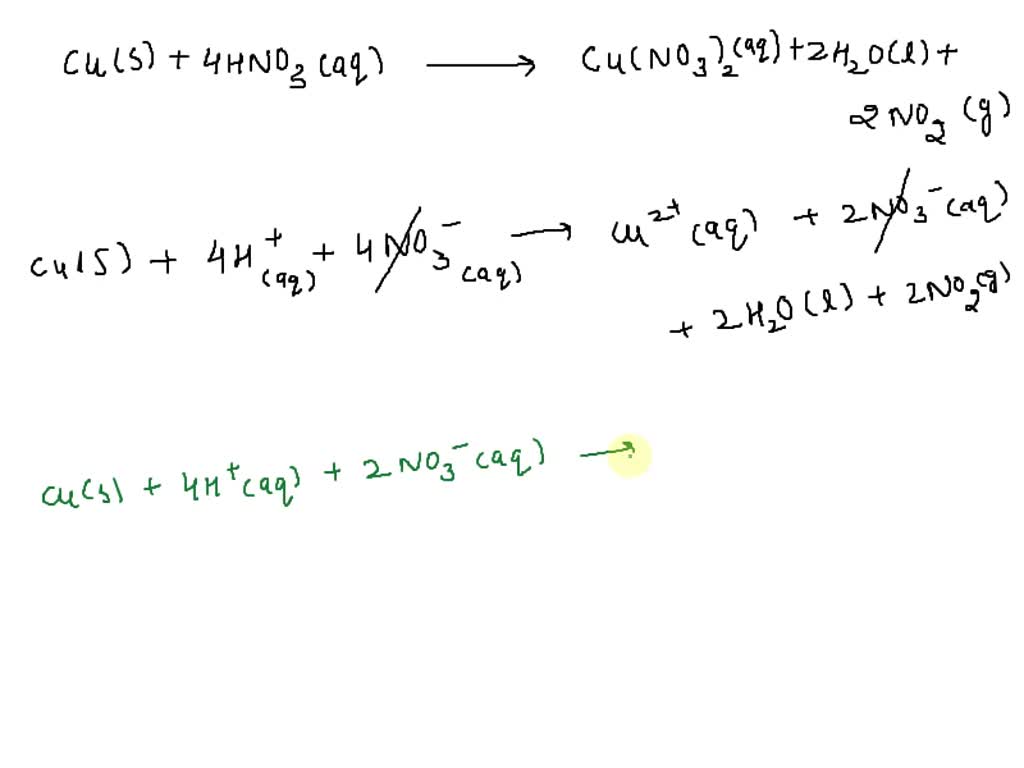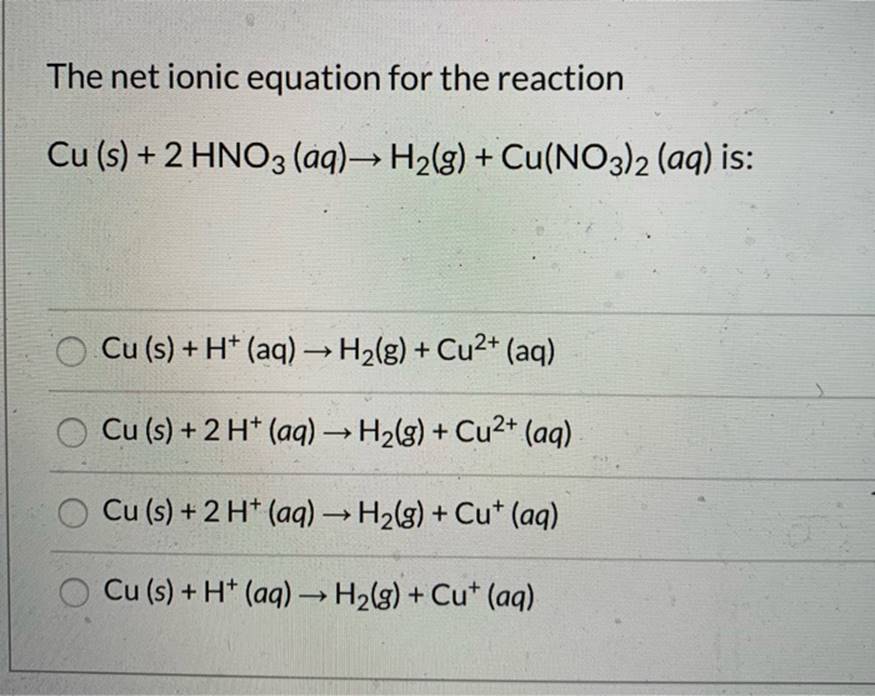
Solved) - The Net Ionic Equation For The Reaction Cu (S) + 2 HNO3 (Aq) ?... (1 Answer) | Transtutors

Balance cu+HNO3= cu(no3)2+No+H2O - Science - Chemical Reactions and Equations - 12587053 | Meritnation.com
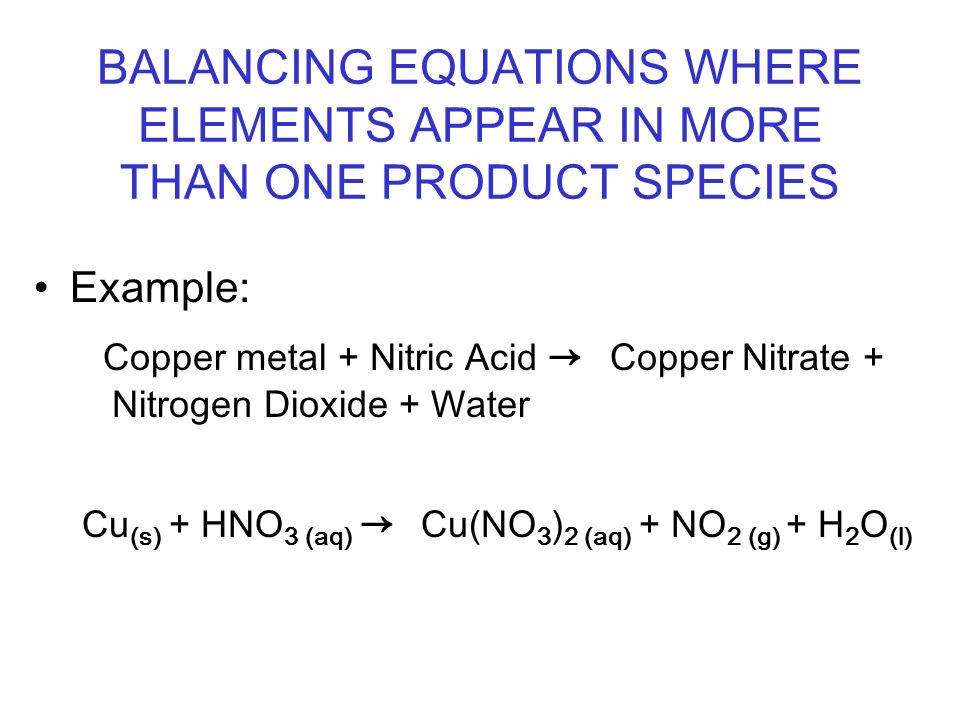
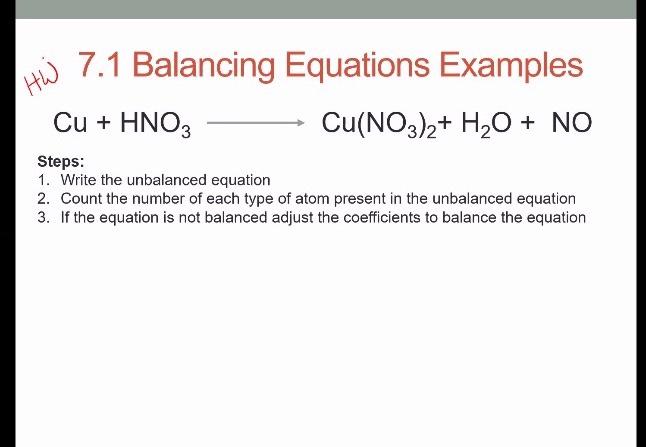
BALANCING EQUATIONS WHERE ELEMENTS APPEAR IN MORE THAN ONE PRODUCT SPECIES Example: Copper metal + Nitric Acid → Copper Nitrate + Nitrogen Dioxide + Water. - ppt video online download

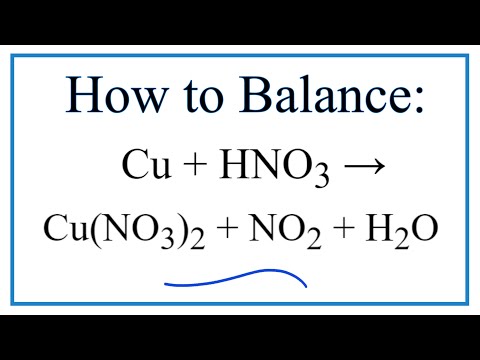
Cu + HNO3 → Cu(NO3)2 + NO2 + H2O ) Balancing Redox Equations Using the Oxidation Number Method - YouTube

W.E-20: Balance the following equation by oxidation number method. Cu + HNO3 – > Cu(NO3)2 + NO2 + H20 Sol. Writing the cridationnumbers ofall the atoms.

Algebraic method or a,b,c method for balance the equation. Cu+HNO3=Cu(NO3)2+NO+H2O balance the equat - YouTube
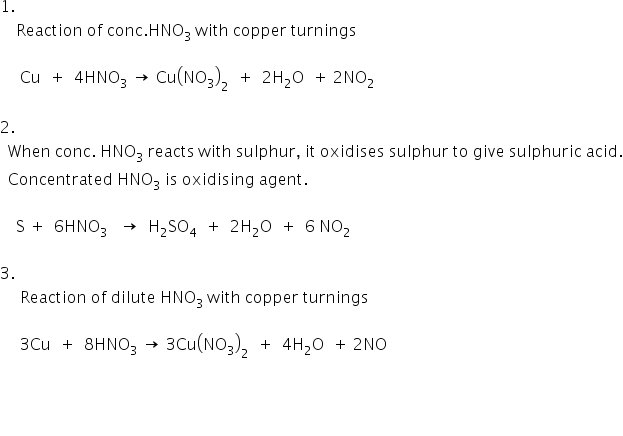
1) write a balanced equation for the reaction of conc.HNO3 when added to copper turnings kept in a beaker.2) write a balanced equation for the reaction of sulphur and hot concentrated nitric

SOLVED: You are given the reaction Cu + HNO3 → Cu(NO3)2 + NO + H2O. Half-reactions: First: 3 upper C u right arrow 3 upper C u superscript 2 plus, plus 6
a Cu + b HNO3 (dil.) —— gt; c Cu (No3)2 + d H2O + e No. How to solve this by algebraic method of balancing?